Eva Farkas
Forsker
Sammendrag
presentation about circular economical projects in NIBIO
Sammendrag
Det er ikke registrert sammendrag
Sammendrag
Det er ikke registrert sammendrag

Divisjon for miljø og naturressurser
AgroComposit
AgroComposit: Biochar-compost composites for supporting site-specific soil agro-ecosystem functions and climate change mitigation

Divisjon for miljø og naturressurser
AgroComposit
AgroComposit: Kompositter av biokull og kompost for å fremme jordas stedsspesifikke agroøkosystemfunksjoner og oppnå utslippskutt

Divisjon for miljø og naturressurser
Biokull-kompostkompositter for å støtte stedsspesifikke agro-økosystemfunksjoner for jord og klimaendringer

Divisjon for miljø og naturressurser
AgriCascade
Cascading recycling of organic N-sources with next-generation biochar fertilizer for Norwegian agriculture

Divisjon for miljø og naturressurser
AgriCascade
AgriCascade: Gjenvinning av organisk nitrogen med forbedret biokull for gjødslings- og miljøgevinster i norsk landbruk
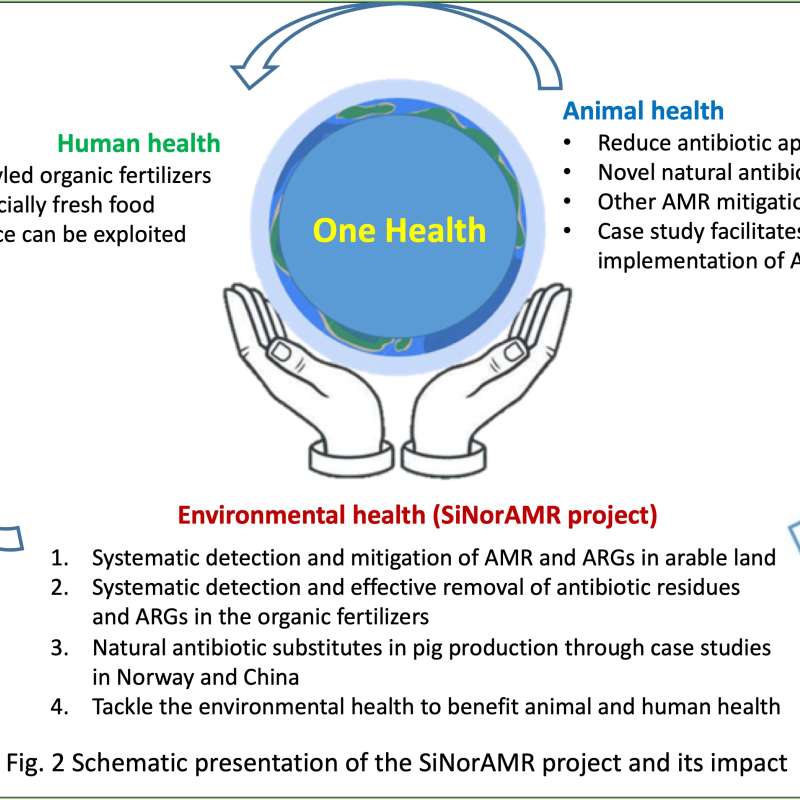
Divisjon for miljø og naturressurser
SiNorAMR
Full title: Collaborative and Knowledge-building Project Collaborative Project Systematic detection and mitigation of antimicrobial resistance in soil environment and animal health contributing to human health (SiNorAMR)
