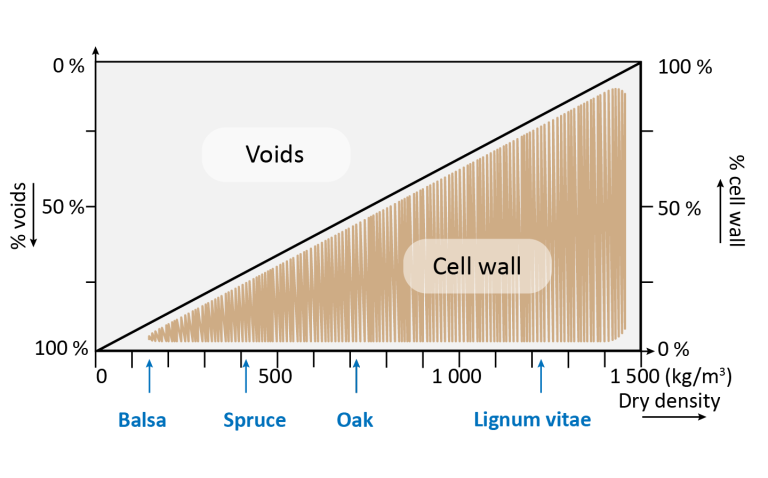
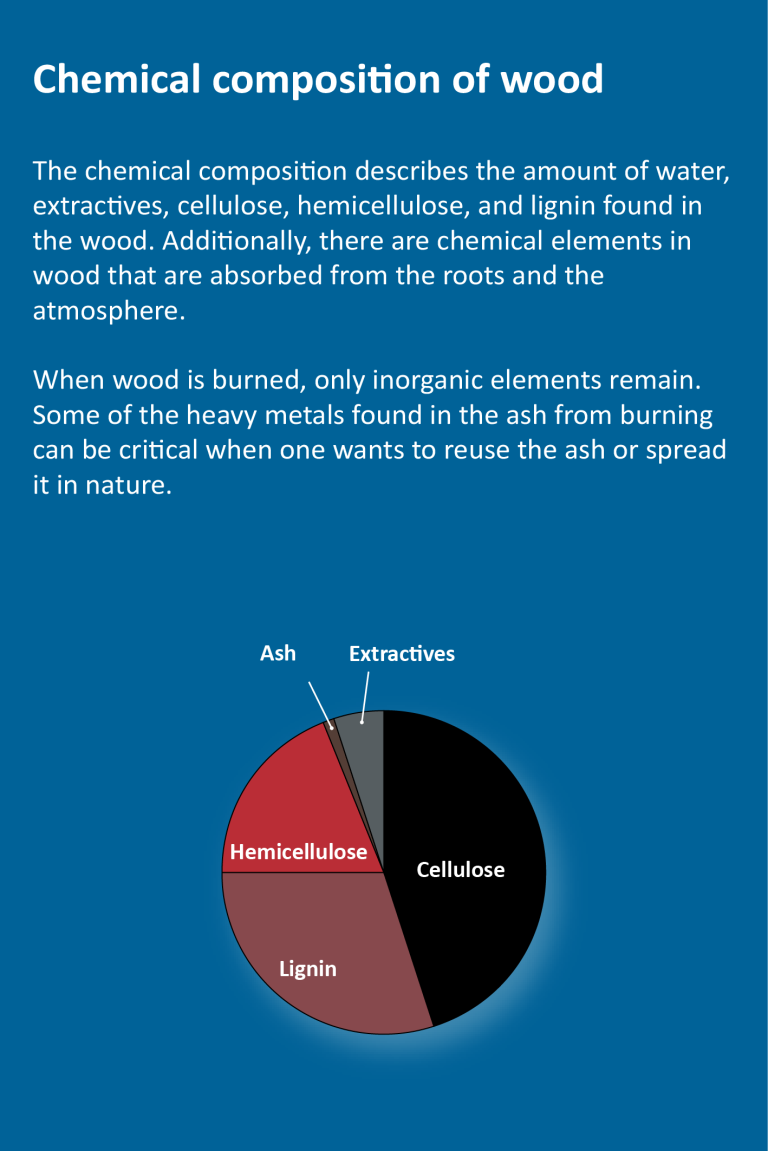
Chemical Composition of Wood
Carbon, hydrogen, and oxygen combine to form the primary organic components of wood: cellulose, hemicellulose, and lignin. These components cannot be easily identified. Separation and quantitative determination of each component can be achieved in the laboratory through the use of solvents or through thermo-gravimetric analysis.
The proportions of cellulose, hemicellulose, and lignin are as follows (in percent of the oven-dry weight of wood):
- cellulose 40-45% (approximately the same in softwoods and hardwoods)
- lignin 25-35% in softwoods and 17-25% in hardwoods
- hemicellulose 20% in softwoods and 15-35% in hardwoods.
Cellulose
Cellulose is composed of glucose molecules (C6H12O6), a monosaccharide produced through photosynthesis from atmospheric carbon dioxide (CO2). These glucose molecules are linked together to form long cellulose chain molecules.
Hemicellulose
Hemicellulose is chemically related to cellulose, as both are carbohydrates. Carbohydrates are chemical substances composed of carbon, hydrogen, and oxygen, with the latter two elements present in the same proportions as in water.
Lignin
Lignin is the cell wall component that distinguishes wood from other cellulosic materials produced by nature. The process of lignification, which involves the deposition of lignin, marks the final stage of cell wall development. Lignin is produced only by living cells. Notably, lignin always occurs in association with cellulose, whereas cellulose can be found almost pure in nature (e.g. in cotton). Lignin is not a carbohydrate. It is predominantly aromatic in nature. The composition of lignin differs between softwoods ("guaiacyl" lignin) and hardwoods ("syringyl" lignin) and also varies especially among different hardwood species.
Tsoumis G. 1991. Science and technology of wood - structure, properties, utilization, 494 pp., Van Nostrand Reinhold, New York.